Case: How we helped a Pharma Company kick off their HFE process
We were approached by a Pharma client that had just completed their clinical trials. Their next step was now to focus on the device that would become the drug user interface - the gatekeeper for a safe, effective and not the least efficient use of their new drug.
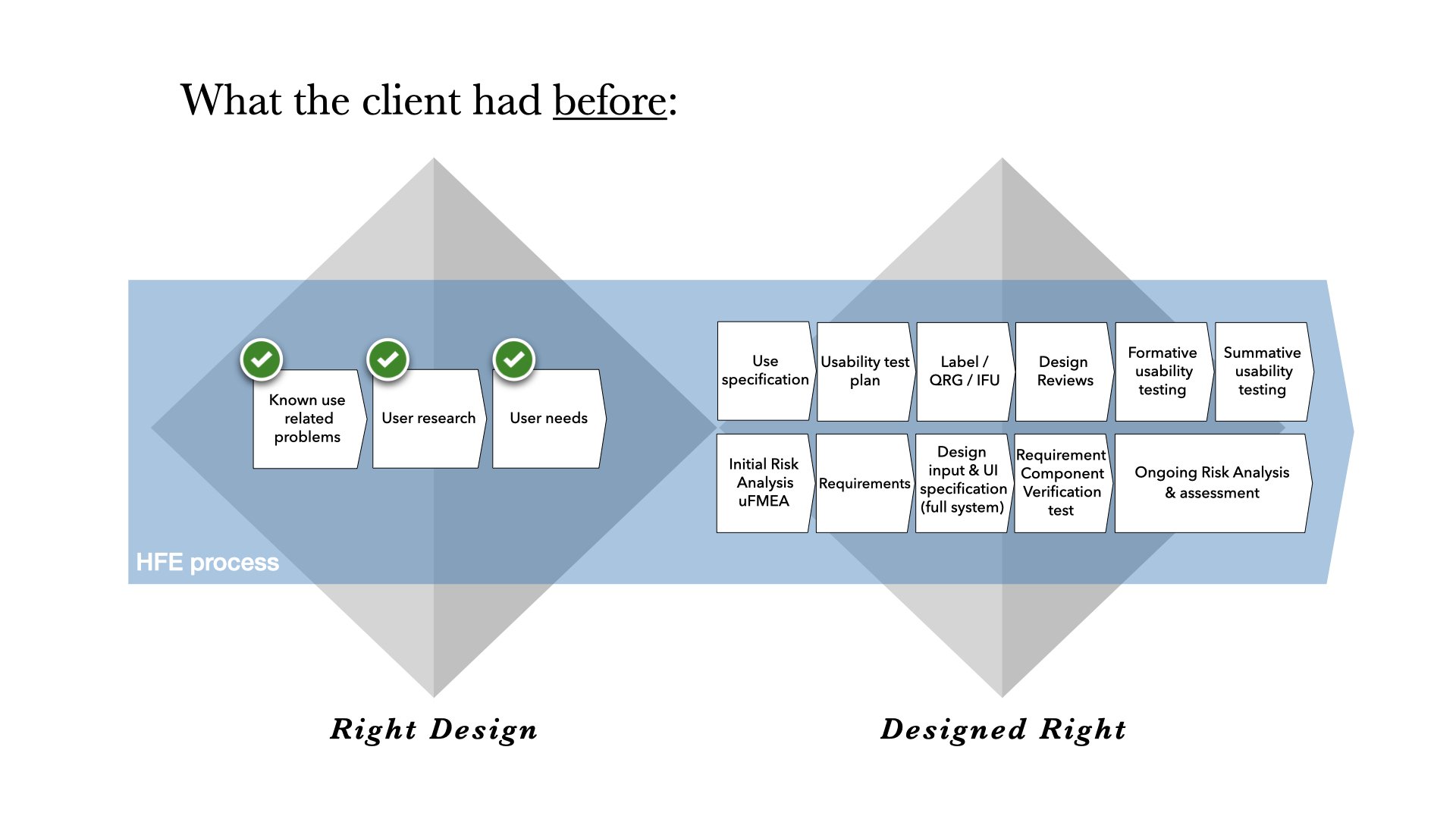
When we first met the client, we unpacked a backlog of their known use-related insights.
For example:
✓ Known use-related problems collected from similar devices on the market.
✓ User research, with close collaboration with field experts and users.
✓ User needs collected by the 3rd. party device developer.
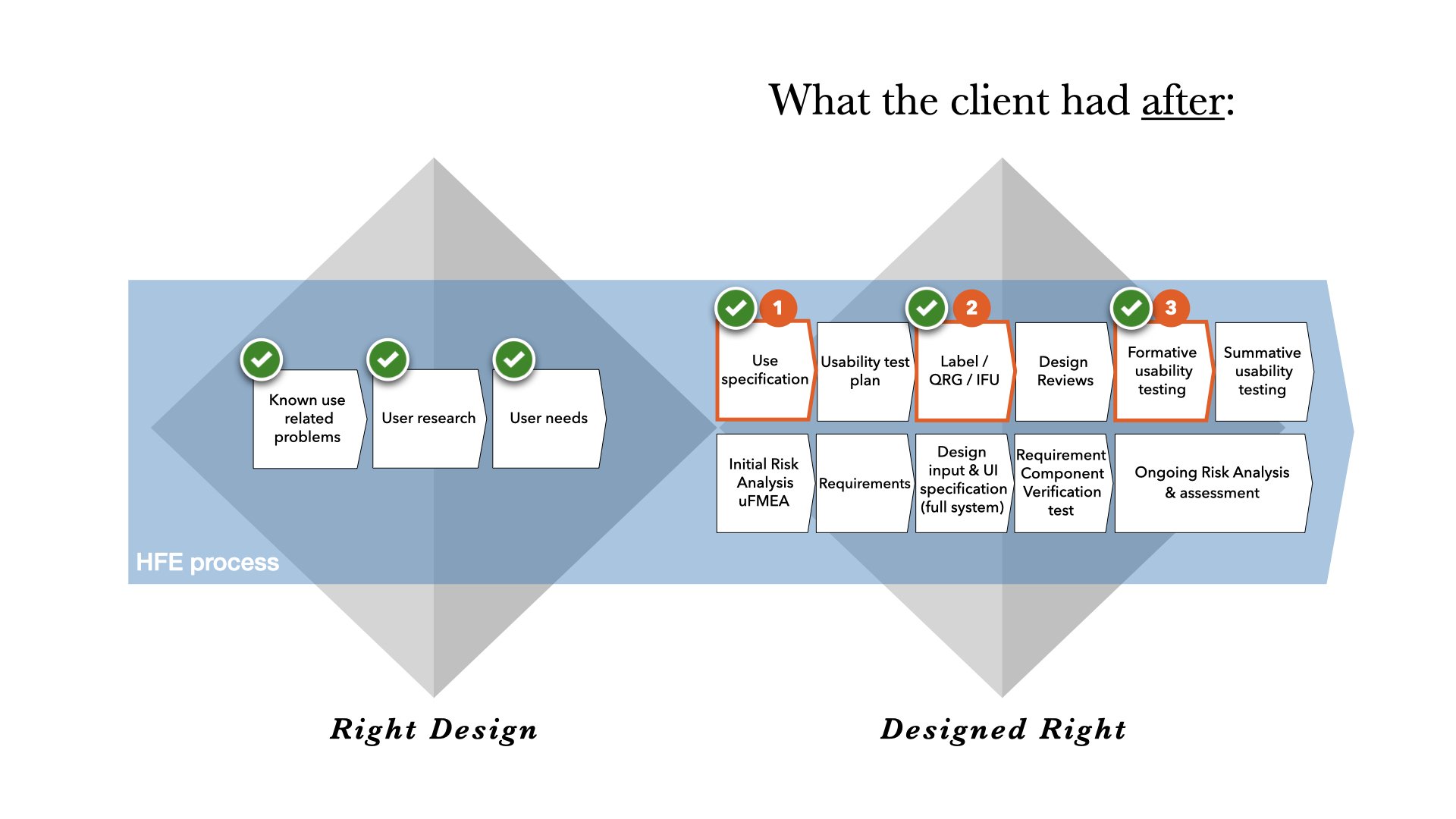
They needed help to structure this knowledge into a format applicable for the EMA/FDA approval. Thus, the first step was to do a Use Specification document.
At the same time, they wanted insights for their Risk Analysis and Usability Test Plan, and therefore they wanted to also carry out the first formal HFE test of the product. Since they did not yet have any instructional material ready at this point, we also provided them with a provisional label and QRG to use in the first formative HFE test.
What our client said
Martin Juhl,
Senior Director of CMC & Device at Cessatech A/S:
“The path to market requires that we obtain a notified body opinion prior to MAA submission; thus we have initiated the early preparation process and have conducted the first Proof-of-Concept usability test at Design Psykologi with the device, evaluating the safety and efficacy of the CT001 device design and user interface according to the task analysis and dosing schedule.
The Proof-of-Concept usability test was a success, and the results were highly valuable for Cessatech and have provided a solid foundation to execute on important future device-related activities to secure a successful market approval.”
The details of how we kicked off the HFE process with the client?
Step 1: How we structured the Use Specification document
In compliance with IEC 62366 standard for the Application of Usability Engineering to Medical Devices, we helped the client structure a use specification that contained:
An intended use statement
A description of intended users and the patient population, including an analysis of the Human Factors attributes of the intended user groups
A description of the intended context of use, including the intended use environment for all intended user groups, and any conditions of use
A task analysis based on the intended use flows, including a Perception, Cognition and Action analysis, success criteria and initial failure modes for all tasks and subtasks
The document was based on user insights and knowledge about user needs that the client had collected from user research. If you have not already collected these insights, we help conduct the user research, or if we discover some gaps in the knowledge about user needs, we can always supplement with residual user research.
Generally, structuring the use specification document is the first step to ensuring that a medical device is safe and effective to use for the intended purposes. The Use specification document is formed at the beginning of product development, but it is often a living document that will be updated over time as the company learn more about the product through the HFE process.
Step 2: How we made a provisional IFU
We provided the client with the layout, copywriting, and pictures for a dummy label and an initial QRG to be used in the formative test. This included the first draft of the label and QRG, and an update of the layout after the client’s review.
We help with all stages of the layout, copywriting and pictures for a label, Quick Reference Guide (QRG) and/or Instructions for Use (IFU) - no matter whether you just want a version to use in the first formative usability test, or if you need help to get the design all the way to its final stage.
Step 3: How we ran the formative usability test
- Study protocol and moderator guide
- Participant recruitment
- Pilot study
- Formative usability test
- Data analysis
- Reporting of findings and recommendations
The next step was to evaluate the safety and efficacy of the device design, label and QRG at their current state. This was done through proven research methods such as simulated use testing, interviews and knowledge-based tasks.
Based on a thorough analysis of the observed behaviour and other insights from the usability test, we provided the client with a set of design guidelines and recommendations for improvements.
We conducted the test in our UX Lab set up with a typical one-way mirror. This allows the client to observe the usability test in real time. We also live-streamed the test, so anyone from the client’s side, who could not be on-site with us, could observe the test from their own office.
As a client, you can be as involved as you want, but we always need you to formally review and approve both the study protocol and the report, as these constitute legal documents in an HFE process. Equally important, they are great tools to ensure in detail that everybody is aligned relative to the why what and how of the test.
Our in-house UX-lab with a one-way mirror
Want to know more?
Read more about what the HFE-package can include, what the exact outcome is, what it can cost and how long it usually takes here.





